Introduction
Drug abuse is a well-known, widespread issue and,
unfortunately, concerns mainly young individuals. According to the
European Monitoring Centre for Drugs and Drug Addiction, in 2017,
cannabis users in Europe were estimated to be approximately 23,5
million, cocaine users approximately 3.5 million and opiate users
approximately 13.5 million. From the opiate users estimated in
2015, only 38% of these asked for help for detoxification (1). Drug rehabilitation programs aim for
the social reintegration of drug-addicted individuals and are based
on two methods: The first method is what is termed as ‘dry’
programs, in which the drug abuser receives psychological support
and assistance, understands the problem and abstains from any
psychoactive substance. The user covers the one third of
rehabilitation programs among the member countries of the European
Union. The second method is substitution treatment programs in
which opioid substances, such as methadone (formerly used) and
buprenorphine (currently used) are administered as substitutes for
the use and abuse of opiates (2).
This type of detoxification covers the remaining 2/3 of
rehabilitation programs (3).
According to the World Health Organization (WHO),
psychoactive substances affect the central nervous system, alter
the emotional state of the individual and cause mental or physical
dependence (4,5). Several studies have highlighted the
effects of these substances on brain activity, increased oxidative
burden, behaviour and accelerated biological aging of therelevant
population (6-8).
One of the cellular aging indexes is the reduction
of telomere length. Telomeres are a repetitive 5'-TTAGGG-3' DNA
sequence at the end of each chromosome, coated by a complex of six
proteins termed ‘shelterin’ proteins [telomeric repeat factor
(TRF)1, TRF2, protection of telomeres protein 1 (POT1),
TERF1-interacting nuclear factor 2 (TIN2), repressor/activator
protein 1 (Rap1) andtripeptidyl-peptidase 1 (TPP1)] (9). These proteins protect the chromosome
ends from double-stranded DNA breaks, deletions, inversions and
translocations leading to eukaryotic chromosomal stability
(10,11). The maintenance of telomere length
depends on an enzyme, known as telomerase, which has the ability of
a cellular reverse transcriptase and is produced in certain levels
in the majority of somatic cells (12). In each cell division, DNA
polymerases cannot maintain telomere repeats and telomere length
becomes increasingly shorter (13).
In addition to the above-mentioned findings,
telomere length has been reported to be a biomarker of cellular
aging, associated with age-related diseases, such as diabetes,
Alzheimer's disease, hypertension and cancer, as well as with a
poor survival (14). It is
estimated that telomere length decreases at a rate of 20-40 base
pairs each year (15), and
factors such as smoking, obesity, a poor diet, drugs, chronic
inflammation, oxidative stress, can lead to a further reduction in
telomere length. The aim of this study was to determine whether
drug abuse can cause a reduction in telomere length, and to
subsequently estimate the percentage of cellular aging.
Materials and methods
Sampling
Blood samples were collected from 16 drug abusers,
all of whom were under a ‘dry’ detoxification program at the
Therapeutic Center for Persons with Addiction (KETHEA). Blood
samples were received within the first 15 days following the
integration of the participants to the detoxification program. As a
control group, we used 70 healthy individuals who voluntarily
attended the Laboratory of Toxicology, Medical School, University
of Crete (Crete, Greece). A written informed consent form was
completed by all participants. This study was approved by the
Ethics Committee of the University of Crete (07/01.11.2018).
Quantitative fluorescent in situ
hybridization (Q-FISH)analysis
Peripheral blood samples (2.5 ml) were collected
from all the participants. Leukocytes, from all samples, were
isolated and cultured in RPMI-1640 culture medium supplemented with
10% fetal bovine serum, 1% L-glutamine, 1% penicillin and
streptomycin for 72 h in a CO2 incubator. Chromosome
preparation was performed using standard methods. Briefly, the
leukocytes were treated with a hypotonic solution and fixed using
an acetic acid:methanol ratio of 1:3. A few drops of fixative
solution containing cells were used for each slide. The slides were
dried and incubated on a hot plate (55˚C) overnight prior to
hybridization. Slides with chromosome preparations were hybridized
with a (C3TA2)3 peptide nucleic acid (PNA) probe (PANAGENE,
Daejeon, Republic of Korea). Following hybridization, digital
images were acquired using a Leica LCS Lite Confocal Microscope
(Leica Microsystems, Wetzlar, Germany). Image acquisition was
performed with a charge-coupled device camera (HCX PL APO CS 63X
objective; Leica Microsystems, Wetzlar, Germany) at a 1,024x1,024
pixel resolution and 8-bit depth. Images were processed with Leica
Q-FISH software, and telomere fluorescence intensity was analyzed
using ImageJ software (https://imagej.nih.gov/ij/) (Fig. 1). To ensure that these Q-FISH
experiments were reproducible, we used the L5178Y-S cell line (cat.
no. 93050408; Culture Collections, Public Health England,
Salisbury, UK) with a stable and known mean telomere length, which
was estimated to be approximately 7 kb (16).
Statistical analysis
The telomere length for whole chromosomes (TL) and
the telomere length from the short telomeres (TL <20th
percentile; TLS were estimated through Q-FISH fluorescence data.
Descriptive statistics, such as the means, percentiles and
quartiles were calculated using a newly developed spreadsheet
termed BIOTEL, which specializes in such calculations (Fig. 2). Additional analyses were
performed using IBM SPSS Statistics 24.0. The associations of
discrete data were made using Pearson's Chi-square test.
Differences in TL or TLS between the controls and abusers were
tested with an independent samples t-test. Pearson's correlation
coefficient (Pearson's rho) was used for correlation coefficient
calculations. A value of P<0.05 was set for the acceptance of
the null hypotheses.
Results
A total of 16 participants, both heavy cannabis and
opiate users and 70 healthy individuals participated in the current
study. From each participant, we collected images from different
metaphases, where the specific binding of the PNA probe in the
telomeric ends is clearly visible. The intensity of the signal of
all telomeric measurements, in all metaphases of each participant,
in combination with the intensity of cell line will be used for the
determination of telomere length (Fig. 1).
The mean age of the drug abusers was 36.7±8.9 years
old, ranging from 24 to 63 years, whereas the control group
adjusted with this group (P=0.136) had a mean age of 41.2±11.2
years. The same adjustment was found when the participants were
divided into 10-year age groups (P=0.287). In addition, sex
distribution differed significantly since only 1 female was present
(6.25%) in the drug abusers group (P<0.001; Table I).
 | Table IDemographic data of the participants
in the study and the control group. |
Table I
Demographic data of the participants
in the study and the control group.
| | Control (n=70) | Drug abusers
(n=16) | |
|---|
| Participant
group | Mean | SD | Mean | SD | P-value |
|---|
| Agea (years) | 41.2 | 11.2 | 36.7 | 8.9 | 0.136 |
|---|
| | Control (n=70) | Drug abusers
(n=16) | |
|---|
| Age
groupsb | n | % | n | % | |
|---|
| 20-30 years | 10 | 76.9 | 3 | 23.1 | 0.287 |
| 30-40 years | 24 | 72.7 | 9 | 27.3 | |
| 40-50 years | 26 | 89.7 | 3 | 10.3 | |
| 60-70 years | 10 | 90.9 | 1 | 9.1 | |
| Sex (male) | 33 | 68.8 | 15 | 31.2 | <0.001 |
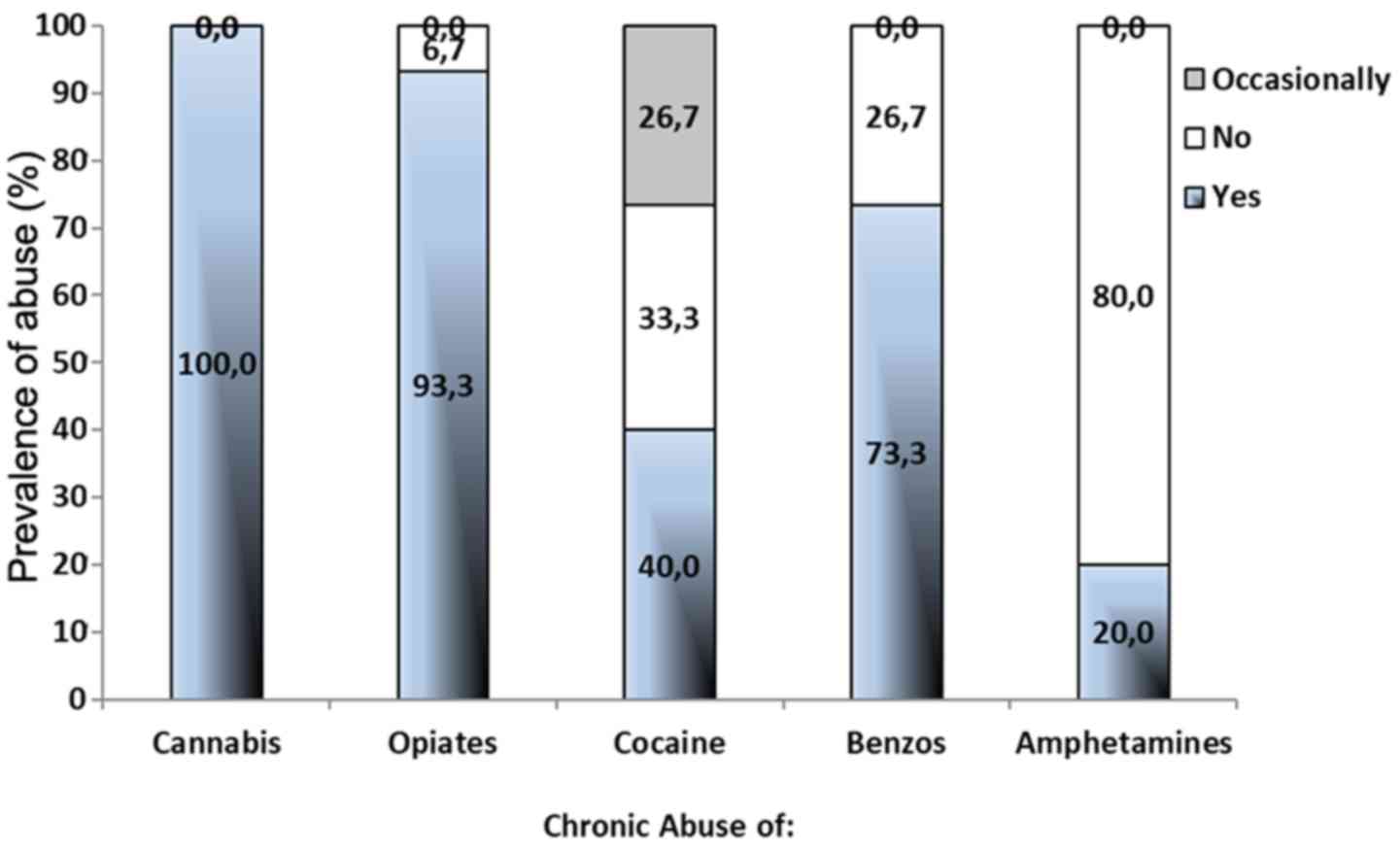
The prevalence of drug abuse is depicted in Fig. 3. All participants had a systematic
abuse of cannabis (100%), while 93.3, 73.3, 40.0 and 20.0% of the
abusers reported a systematic use of opiates, benzodiazepines,
cocaine and amphetamines, respectively. Fig. 4 represents the years of chronic
abuse of the reported drugs. More specifically, cannabis abusers
exhibited a mean of 14.1±6.2, opiate abusers a mean of 11.6±7.1
years, cocaine abusers a mean of 9.0±4 years, benzodiazepine
abusers a mean of 9.6±9.0 years, and amphetamines abusers a mean of
6.0±1.7 years.
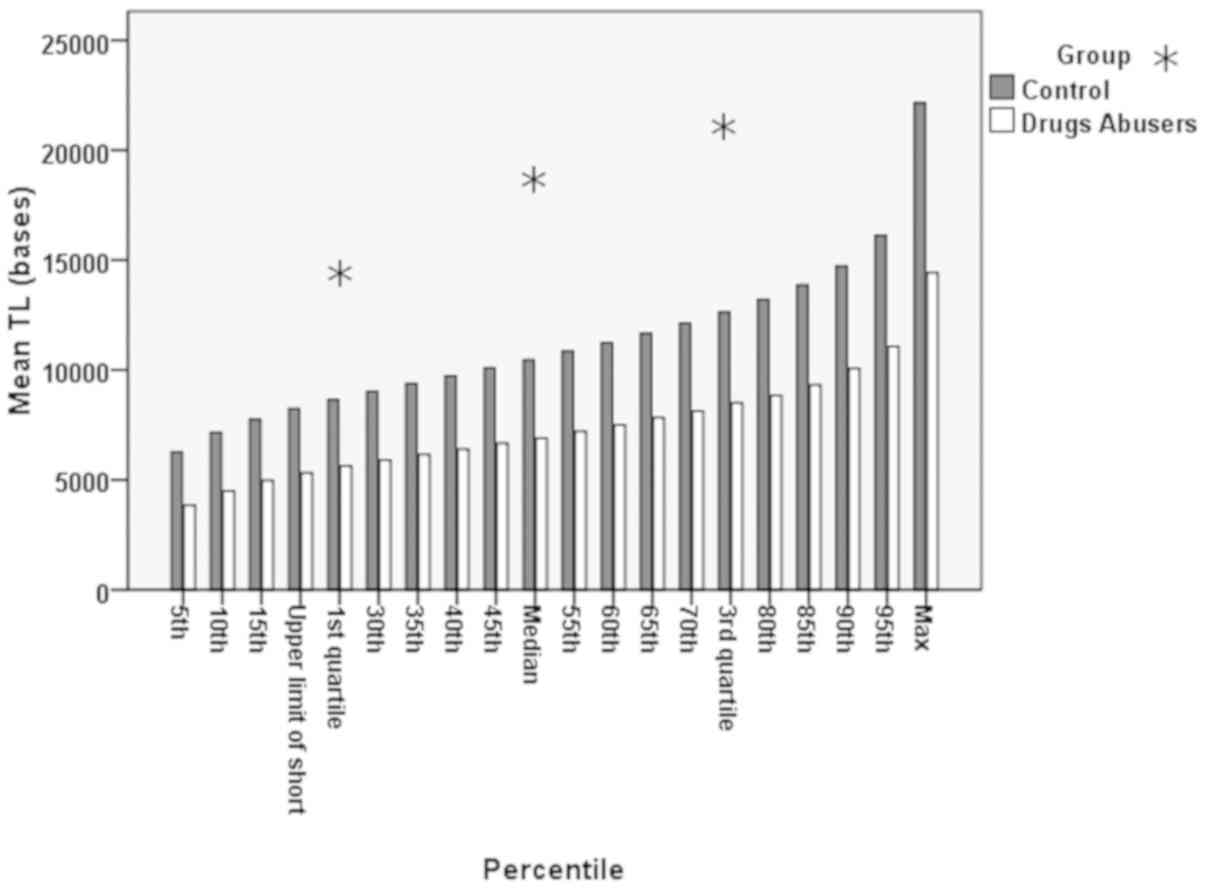
Bar charts of quartiles per the 5 percentile
increment between the drug abusers and the control group are shown
in Fig. 5. In Table II, the representation of a
comparison between the quartiles of TL from the whole and from the
short telomeres (TLS) (<20% of TL) between the drug abusers and
the controls is presented. Additionally, in Fig. 5, it can be seen that although the
pattern of increase was similar between the 2 groups, there was a
definite distance, a statistically significant difference in the TL
(P<0.001).
 | Table IIDescriptive statistics of TL and TLS
quartiles (expressed as bases) between the drug abusers and the
control group. |
Table II
Descriptive statistics of TL and TLS
quartiles (expressed as bases) between the drug abusers and the
control group.
| | | Control (n=70) | Drug abusers
(n=16) | |
|---|
| | Quartile | Mean | SD | Median | Mean | SD | Median | P-value |
|---|
| TL | 1st | 8,656 | 1,506 | 8,515 | 5,631 | 2,511 | 4,709 | <0.001 |
| | Median | 1,0469 | 1,783 | 1,0472 | 6,901 | 3,283 | 5,649 | <0.001 |
| | 3rd | 12,630 | 2,214 | 12,876 | 8,506 | 4,246 | 6,571 | <0.001 |
| TLS | 1st | 6,270 | 1,291 | 6,344 | 3,818 | 2,059 | 3,301 | <0.001 |
| | Median | 7,155 | 1,353 | 7,121 | 4,450 | 2,121 | 3,959 | <0.001 |
| | 3rd | 7,750 | 1,412 | 7,640 | 4,961 | 2,137 | 4,340 | <0.001 |
Moreover, Table
II indicates that at all examined quartiles, the TL and TLS
were estimated to be significantly higher in the controls than in
the drug abusers (P<0.001). In particular, for the median TLS,
which is a marker of biological age, the control groups exhibited a
mean of median 7,155±1,353 bases, higher than that of the drug
abusers (4,450±2,121 bases).
Finally, the years of chronic abuse of cannabis and
opiates did not significantly correlate with the median TLS. The
estimated correlation values between the median TLS with the years
of cannabis abuse were (rs=-0.030, P=0.912) and median TLS
(rs=-0.399, P=0.158) with opiate abuse (Fig. 6).
Discussion
In the current study, we examined the association
between the length of telomeric repeats of the DNA and the actual
biological age of each individual. It was found that there was a
statistically significant difference in telomere length between
abusers and the control group (healthy individuals). This led us to
the conclusion that the actual biological age of the abusers was
far greater than their chronological age, which possibly classifies
these psychotropic substances (opiates and cannabinoids) into
exogenous factors that lead to the premature biological aging of
the organism.
More specifically, with regard to the statistical
distribution of telomere length, the distribution of the abusers
had the same pattern with that of the controls (Fig. 5), although a statistically
significant reduction in the median value of the telomere length of
the abusers was observed. In addition, we can evaluate the
reduction in telomere length by the fact that it has been proven
bibliographically that short telomeres play a major role in the
reduction of telomere length. As the number of shorter telomeres
increases the total telomere length decreases and this is related
to cellular aging (17-22).
Furthermore, it is important to mention the
association between telomere length and the years of drug abuse. As
illustrated in Fig. 6, a
specific, yet not significant trend was observed between telomere
length and the years of both cannabis and opiates abuse. More
specific, the mean TL of the drug users appeared to decrease as the
year of abuse increased, and this result was more evident in the
opiate abusers. Depending on previous findings, which demonstrated
a downward trend as the years of opiate abuse increased (23), there is a clear need for further
investigation of this issue. The non-association of the years of
abuse and TL could possibly be affected by the limited number of
participants.
Over the past few years, research on telomere length
has intensified, with the majority of scientists considering
telomere length in relation to various diseases, such as cancer,
diabetes and various cardiovascular diseases (24-35).
In addition, there are some studies which have associated various
psychotropic substances, such as anxiolytics, antidepressants and
hypnotics with TLS (36-47).
These investigations, revealed in a more general manner, the
association of telomere length with the above-mentioned factors,
although they did not associate it with biological age
specifically.
The findings of this study are in accordance with
those of the study by Imam et al which provided an
association between drug abuse and telomere length shortening
(48). In the study by Yang et
al, was shown that heroin and diazepam abusers displayed
shorter long telomere lengths (LTLs) compared to those who used
other drugs, probably due to the increase in oxidative stress.
Furthermore, they discovered a significant negative correlation
between the LTL and the length of time before relapse (23). In the same study, no significant
difference in telomere length was depicted between drug abusers
using intravenous injection and those using other methods (e.g.,
smoking) (23).
It has been shown that oxidative stress is one of
the major factors that lead to the reduction in telomere length
with increasing age. An increase in oxidative stress results in a
decreased telomerase activity (49,50), a reduced cell proliferation and
increased apoptosis (18,20). This has been confirmed by the fact
that when oxidative stress is suppressed by various factors, such
as an increased concentration of vitamin C, the reduction in
telomere length is limited (51).
Thus, it is very likely that the reduction in telomere length in
drug abusers occurs due to the high levels of oxidative stress
generated by the psychotropic substances.
In conclusion, the present study demonstrates that
there is an association between telomere length and drug abuse.
Specifically, it was found that in the drug abusers, the decrease
in telomere was more evident compared to the healthy controls,
indicating that drug abuse does play a role in the shortening of
telomeres. Although usually age is proportional to the years of
chronic abuse, there is no clear evidence of telomere shortening
with the duration of self-referred chronic abuse. However, further
studies are warranted to confirm our findings, using larger samples
sizes of drug abusers.
Acknowledgements
The authors wish to thank the members of the
Therapeutic Center for Persons with Addiction (KETHEA)for their
contribution to blood sampling.
Funding
This study was supported by Toxplus S.A. and the
specific account for research (ELKE) of the University of Crete (KA
3464, 3963, 3962).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
EV, MT, DT, DAS and AT were involved in the design
and conception of the study. EV, MT, DN, DT and KKan were involved
in blood sampling. EV, KT, PF, KKal, GV and SP were performed the
analysis of the blood samples following the Q-FISH protocol. EV,
MT, KT, PF, DT and AA wrote the manuscript. EV and AA were involved
in the statistical analysis of the results and to designing the
figures and tables. EV, MT, KT, PF, DT, AA, DAS and AT were
involved in the proofreading and editing if the manuscript. All
authors have taken the responsibility for publishing this study and
all authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
AT is affiliated with Toxplus S.A. and that this
company also provided funding for this study. The information and
views set out in this study are those of the authors and do not
necessarily reflect the official opinion of Toxplus S.A. Toxplus
S.A. may not be held responsible for the use which may be made of
the information contained herein. DAS is the Managing Editor of the
journal, but had no personal involvement in the reviewing process,
or any influence in terms of adjudicating on the final decision,
for this article. The other authors declare that there are no
conflicts of interest.
References
|
1
|
European Monitoring Centre for Drugs and
Drug Addiction (EMCDDA): http://www.emcdda.europa.eu/data/stats2017_en.
Accessed January 23,2019.
|
|
2
|
Tzatzarakis MN, Vakonaki E, Kovatsi L,
Belivanis S, Mantsi M, Alegakis A, Liesivuori J and Tsatsakis AM:
Determination of buprenorphine, norbuprenorphine and naloxone in
fingernail clippings and urine of patients under opioid
substitution therapy. J Anal Toxicol. 39:313–320. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diaper AM, Law FD and Melichar JK:
Pharmacological strategies for detoxification. Br J Clin Pharmacol.
77:302–314. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
WHO Expert Committee on Drug Dependence:
Thirty-eighth report. In: WHO Technical Report Series. WHO,
Switzerland. 2017.
|
|
5
|
Nestoros JN, Vakonaki E, Tzatzarakis MN,
Alegakis A, Skondras MD and Tsatsakis AM: Long lasting effects of
chronic heavy cannabis abuse. Am J Addict. 26:335–342.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheng GL, Zeng H, Leung MK, Zhang HJ, Lau
BW, Liu YP, Liu GX, Sham PC, Chan CC, So KF, et al: Heroin abuse
accelerates biological aging: A novel insight from telomerase and
brain imaging interaction. Transl Psychiatry.
3(e260)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ersche KD, Jones PS, Williams GB, Robbins
TW and Bullmore ET: Cocaine dependence: A fast-track for brain
ageing? Mol Psychiatry. 18:134–135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bachi K, Sierra S, Volkow ND, Goldstein RZ
and Alia-Klein N: Is biological aging accelerated in drug
addiction? Curr Opin Behav Sci. 13:34–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vakonaki E, Tsiminikaki K, Plaitis S,
Fragkiadaki P, Tsoukalas D, Katsikantami I, Vaki G, Tzatzarakis MN,
Spandidos DA and Tsatsakis AM: Common mental disorders and
association with telomere length. Biomed Rep. 8:111–116.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Lange T: Shelterin: The protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chan SW and Blackburn EH: New ways not to
make ends meet: Telomerase, DNA damage proteins and
heterochromatin. Oncogene. 21:553–563. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Calado RT and Young NS: Telomere diseases.
N Engl J Med. 361:2353–2365. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Starkweather AR, Alhaeeri AA, Montpetit A,
Brumelle J, Filler K, Montpetit M, Mohanraj L, Lyon DE and
Jackson-Cook CK: An integrative review of factors associated with
telomere length and implications for biobehavioral research. Nurs
Res. 63:36–50. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
McIlrath J, Bouffler SD, Samper E,
Cuthbert A, Wojcik A, Szumiel I, Bryant PE, Riches AC, Thompson A,
Blasco MA, et al: Telomere length abnormalities in mammalian
radiosensitive cells. Cancer Res. 61:912–915. 2001.PubMed/NCBI
|
|
17
|
Armanios M, Alder JK, Parry EM, Karim B,
Strong MA and Greider CW: Short telomeres are sufficient to cause
the degenerative defects associated with aging. Am J Hum Genet.
85:823–832. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Blasco MA: Telomere length, stem cells and
aging. Nat Chem Biol. 3:640–649. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hao LY, Armanios M, Strong MA, Karim B,
Feldser DM, Huso D and Greider CW: Short telomeres, even in the
presence of telomerase, limit tissue renewal capacity. Cell.
123:1121–1131. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hemann MT, Strong MA, Hao LY and Greider
CW: The shortest telomere, not average telomere length, is critical
for cell viability and chromosome stability. Cell. 107:67–77.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rajaraman S, Choi J, Cheung P, Beaudry V,
Moore H and Artandi SE: Telomere uncapping in progenitor cells with
critical telomere shortening is coupled to S-phase progression in
vivo. Proc Natl Acad Sci USA. 104:17747–17752. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Samper E, Flores JM and Blasco MA:
Restoration of telomerase activity rescues chromosomal instability
and premature aging in Terc-/- mice with short telomeres. EMBO Rep.
2:800–807. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang Z, Ye J, Li C, Zhou D, Shen Q, Wu J,
Cao L, Wang T, Cui D, He S, et al: Drug addiction is associated
with leukocyte telomere length. Sci Rep. 3(1542)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Willeit P, Willeit J, Brandstätter A,
Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl
M, Kronenberg F, et al: Cellular aging reflected by leukocyte
telomere length predicts advanced atherosclerosis and
cardiovascular disease risk. Arterioscler Thromb Vasc Biol.
30:1649–1656. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Demissie S, Levy D, Benjamin EJ, Cupples
LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney
JF, et al: Insulin resistance, oxidative stress, hypertension, and
leukocyte telomere length in men from the Framingham Heart Study.
Aging Cell. 5:325–330. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fitzpatrick AL, Kronmal RA, Gardner JP,
Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M and Aviv A:
Leukocyte telomere length and cardiovascular disease in the
cardiovascular health study. Am J Epidemiol. 165:14–21.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Astrup AS, Tarnow L, Jorsal A, Lajer M,
Nzietchueng R, Benetos A, Rossing P and Parving HH: Telomere length
predicts all-cause mortality in patients with type 1 diabetes.
Diabetologia. 53:45–48. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Olivieri F, Lorenzi M, Antonicelli R,
Testa R, Sirolla C, Cardelli M, Mariotti S, Marchegiani F, Marra M,
Spazzafumo L, et al: Leukocyte telomere shortening in elderly
Type2DM patients with previous myocardial infarction.
Atherosclerosis. 206:588–593. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin TT, Norris K, Heppel NH, Pratt G,
Allan JM, Allsup DJ, Bailey J, Cawkwell L, Hills R, Grimstead JW,
et al: Telomere dysfunction accurately predicts clinical outcome in
chronic lymphocytic leukaemia, even in patients with early stage
disease. Br J Haematol. 167:214–223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gancarcíková M, Zemanová Z, Brezinová J,
Berková A, Vcelíková S, Smigová J and Michalová K: The role of
telomeres and telomerase complex in haematological neoplasia: The
length of telomeres as a marker of carcinogenesis and prognosis of
disease. Prague Med Rep. 111:91–105. 2010.PubMed/NCBI
|
|
31
|
Nzietchueng R, Elfarra M, Nloga J, Labat
C, Carteaux JP, Maureira P, Lacolley P, Villemot JP and Benetos A:
Telomere length in vascular tissues from patients with
atherosclerotic disease. J Nutr Health Aging. 15:153–156.
2011.PubMed/NCBI
|
|
32
|
Saliques S, Zeller M, Lorin J, Lorgis L,
Teyssier JR, Cottin Y, Rochette L and Vergely C: Telomere length
and cardiovascular disease. Arch Cardiovasc Dis. 103:454–459.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ma D, Zhu W, Hu S, Yu X and Yang Y:
Association between oxidative stress and telomere length in type 1
and type 2 diabetic patients. J Endocrinol Invest. 36:1032–1037.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Testa R, Olivieri F, Sirolla C, Spazzafumo
L, Rippo MR, Marra M, Bonfigli AR, Ceriello A, Antonicelli R,
Franceschi C, et al: Leukocyte telomere length is associated with
complications of type 2 diabetes mellitus. Diabet Med.
28:1388–1394. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yan J, Yang Y, Chen C, Peng J, Ding H and
Wen Wang D: Short leukocyte telomere length is associated with
aortic dissection. Intern Med. 50:2871–2875. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wikgren M, Maripuu M, Karlsson T,
Nordfjäll K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson
LG, Adolfsson R and Norrback KF: Short telomeres in depression and
the general population are associated with a hypocortisolemic
state. Biol Psychiatry. 71:294–300. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wolkowitz OM, Jeste DV, Martin AS, Lin J,
Daly RE, Reuter C and Kraemer H: Leukocyte telomere length: Effects
of schizophrenia, age, and gender. J Psychiatr Res. 85:42–48.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Garcia-Rizo C, Fernandez-Egea E, Miller
BJ, Oliveira C, Justicia A, Griffith JK, Heaphy CM, Bernardo M and
Kirkpatrick B: Abnormal glucose tolerance, white blood cell count,
and telomere length in newly diagnosed, antidepressant-naïve
patients with depression. Brain Behav Immun. 28:49–53.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fernandez-Egea E, Bernardo M, Heaphy CM,
Griffith JK, Parellada E, Esmatjes E, Conget I, Nguyen L, George V,
Stöppler H, et al: Telomere length and pulse pressure in newly
diagnosed, antipsychotic-naive patients with nonaffective
psychosis. Schizophr Bull. 35:437–442. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Karabatsiakis A, Kolassa IT, Kolassa S,
Rudolph KL and Dietrich DE: Telomere shortening in leukocyte
subpopulations in depression. BMC Psychiatry.
14(192)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Boks MP, van Mierlo HC, Rutten BP,
Radstake TR, De Witte L, Geuze E, Horvath S, Schalkwyk LC, Vinkers
CH, Broen JC, et al: Longitudinal changes of telomere length and
epigenetic age related to traumatic stress and post-traumatic
stress disorder. Psychoneuroendocrinology. 51:506–512.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ramin C, Wang W, Prescott J, Rosner B,
Simon NM, De Vivo I and Okereke OI: A prospective study of
leukocyte telomere length and risk of phobic anxiety among women.
Psychiatry Res. 230:545–552. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Okereke OI, Prescott J, Wong JY, Han J,
Rexrode KM and De Vivo I: High phobic anxiety is related to lower
leukocyte telomere length in women. PLoS One.
7(e40516)2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hartmann N, Boehner M, Groenen F and Kalb
R: Telomere length of patients with major depression is shortened
but independent from therapy and severity of the disease. Depress
Anxiety. 27:1111–1116. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
45
|
Simon NM, Walton ZE, Bui E, Prescott J,
Hoge E, Keshaviah A, Schwarz N, Dryman T, Ojserkis RA, Kovachy B,
et al: Telomere length and telomerase in a well-characterized
sample of individuals with major depressive disorder compared to
controls. Psychoneuroendocrinology. 58:9–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Czepielewski LS, Massuda R, Panizzutti B,
da Rosa ED, de Lucena D, Macêdo D, Grun LK, Barbé-Tuana FM and Gama
CS: Telomere length in subjects with schizophrenia, their
unaffected siblings and healthy controls: Evidence of accelerated
aging. Schizophr Res. 174:39–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Needham BL, Mezuk B, Bareis N, Lin J,
Blackburn EH and Epel ES: Depression, anxiety and telomere length
in young adults: Evidence from the National Health and Nutrition
Examination Survey. Mol Psychiatry. 20:520–528. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Imam T, Jitratkosol MH, Soudeyns H, Sattha
B, Gadawski I, Maan E, Forbes JC, Alimenti A, Lapointe N, Lamarre
V, et al: CIHR Emerging Team Grant on HIV Therapy and Aging: CARMA:
Leukocyte telomere length in HIV-infected pregnant women treated
with antiretroviral drugs during pregnancy and their uninfected
infants. J Acquir Immune Defic Syndr. 60:495–502. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Epel ES, Blackburn EH, Lin J, Dhabhar FS,
Adler NE, Morrow JD and Cawthon RM: Accelerated telomere shortening
in response to life stress. Proc Natl Acad Sci USA.
101:17312–17315. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wolkowitz OM, Epel ES, Reus VI and Mellon
SH: Depression gets old fast: Do stress and depression accelerate
cell aging? Depress Anxiety. 27:327–338. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Furumoto K, Inoue E, Nagao N, Hiyama E and
Miwa N: Age-dependent telomere shortening is slowed down by
enrichment of intracellular vitamin C via suppression of oxidative
stress. Life Sci. 63:935–948. 1998.PubMed/NCBI View Article : Google Scholar
|